
About I-SPY 2
For patients enrolled before June 23, 2022
Who can be screened for I-SPY 2 Trial eligibility?
Women and men who are:
- At least 18 years old
- Have a new diagnosis of Stage 2 or 3 invasive breast cancer
- Have a tumor 2.5cm (almost an inch) or larger
If you meet these three requirements, no matter what type of invasive breast cancer you have and whether or not there is cancer in your lymph nodes, you are eligible to be screened for participation in the I-SPY 2 Trial.
During the Screening Phase your study doctor will check to make sure it is safe for you to take part in the study. This includes checking your heart, liver and kidney function. You will have a biopsy (tissue sample of your tumor) and a test called MammaPrint®. MammaPrint is a test to find out if your cancer would be at high risk for recurrence if the only treatment you had for your cancer was surgery.
You can continue on to the treatment phase if you:
- are otherwise healthy
- have not had cancer within the last 5 years*
- have not had chemotherapy for this breast cancer
- are not pregnant or breastfeeding
* You may still participate if you have had breast cancer, carcinoma in situ of the cervix, colon or rectum, melanoma in situ, basal cell and squamous cell carcinomas of the skin, or papillary thyroid cancer within the last 5 years.
Estrogen Receptor (ER) status, Progesterone Receptor (PR) status, and HER2 receptor status are common biomarkers used to characterize a breast cancer. These biomarkers will be used, along with the MammaPrint test score to help determine whether you are eligible to join the Chemotherapy or the Endocrine Treatment Phase of the trial.
You are eligible to continue onto the Chemotherapy Treatment Phase, if your cancer matches one of these descriptions:
Estrogen Receptor positive & MammaPrint High Risk
Estrogen Receptor negative
HER2 positive
You are eligible to continue onto the Endocrine Treatment Phase, if your cancer matches this description:
Estrogen Receptor positive, HER2 negative & MammaPrint Low Risk
You can find more information about the Endocrine Treatment Phase here.
What to expect in each phase of the trial
The timeline below shows the activities that occur during each phase of the trial, depending on whether you are receiving Chemotherapy or Endocrine therapy.
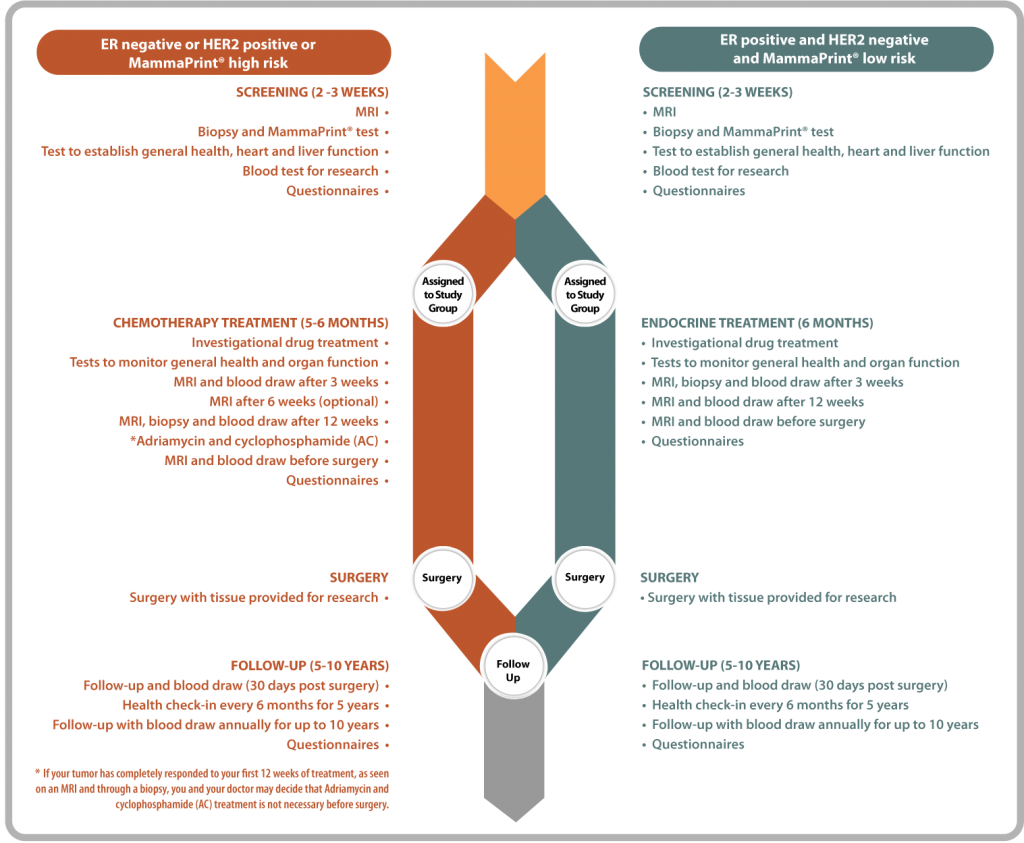
Why consider joining the I-SPY 2 Trial?
The I-SPY 2 Trial treats newly diagnosed breast cancer with neoadjuvant drug therapy. This means that drug therapy is given before surgery. Neoadjuvant therapy has advantages and disadvantages. One advantage is that it may shrink your tumor, allowing you to have a smaller surgery. One disadvantage is that you have to wait until you have finished neoadjuvant therapy to have your surgery, which may be stressful for some people. However, it is just as safe and effective to have therapy before surgery as after surgery. You can learn more about the pros and cons of neoadjuvant therapy here.
The I-SPY 2 Trial is testing new investigational drugs and combinations of drugs to find out whether they are better, worse or no different than the standard treatment. Most patients on the trial receive an investigational drug or drug combination that would not be available to them as standard treatment.
The I-SPY 2 Trial uses MRIs to monitor your tumor. MRI (Magnetic Resonance Imaging) scans provide you and your care team with detailed pictures of your tumor. Your doctor can see if your tumor is responding to the drugs or not. Your treatment plan could be changed if your tumor is not responding.
You will have one MRI during the screening phase of the study. You will have another MRI three weeks after you start treatment and again when you complete the first twelve weeks of treatment. You will have your final MRI before you have surgery.
The I-SPY 2 Trial measures biomarkers (characteristics of your tumor) to learn as much as possible about your breast cancer and how it responds to treatment. A small part of your tumor will be collected by biopsy. Part of this biopsy will be used for molecular testing. These tests may give you and your doctor more information about your tumor, which will help with planning your treatment.
What drugs are used in the I-SPY 2 Trial?
(Additional information on common drugs mentioned can be found on the Cleveland Clinic’s drug information site: Chemocare.com)
Information for I-SPY 2 Patients Who Are Eligible for Chemotherapy Treatment
Standard Chemotherapy Treatment
The I-SPY 2 trial uses the standard treatment as the comparison (control) group. If your breast cancer is HER2 negative the standard treatment is twelve weekly doses (referred to as cycles) of paclitaxel followed by four cycles of Adriamycin and cyclophosphamide (AC). If your breast cancer is HER2 positive the standard treatment includes trastuzumab +/- pertuzumab as well as twelve weekly doses of paclitaxel followed by four cycles of Adriamycin and cyclophosphamide.
Paclitaxel is a drug that stops cancer cells from dividing and causes them to die. It does this by limiting the cells ability to divide in two.
Adriamycin and cyclophosphamide also stop cells from dividing and cause them to die, but they do this by tangling with the DNA to damage it. Using the paclitaxel followed by Adriamycin and cyclophosphamide has been shown to be an effective treatment for many patients with breast cancer.
Trastuzumab and pertuzumab are HER2 targeted therapies that can slow or stop the growth of HER2 positive breast cancer.
Investigational Drugs
The investigational drugs in the I-SPY 2 trial are being tested to find drugs that are better than the standard treatment that is currently given to patients. We are also looking for drugs that are at least as good as the standard treatment but with fewer side effects. In addition, we are looking for biomarkers that could be used to select the most effective drug for each patient with the least side effects. In other words – the right drug for the right patient.
The investigational drugs available in the trial change over time. There are usually three or more drug treatments being tested but you or your doctor will not choose which one you get. A computer will randomly assign the drugs to you. This is the most scientific way to reduce bias in a clinical trial. Information about your tumor and what we have learned from prior I-SPY 2 patients that have received each drug, will affect how the computer assigns drugs to you. This means that if an investigational drug is showing benefit to I-SPY 2 patients with tumors similar to yours, you will have an increased chance of receiving that drug.
Once you are assigned to a drug treatment, your study team will explain how the drug treatment works and what side effects you could experience. You can then choose to continue with the treatment phase of the trial or not.
Information For I-SPY 2 Patients Who Are Eligible For Endocrine Treatment
For women with estrogen receptor positive breast cancer the standard treatment is anti-estrogen therapy, for example Tamoxifen or aromatase inhibitors (AIs). These work by either blocking estrogen from attaching to the breast cancer cells or by lowering the amount of estrogen in the body.
There are new anti-estrogen drugs being tested called Selective Estrogen Receptor Degraders, or SERDs, that block the effects of estrogen in breast tissue and also reduce the number of estrogen receptors. SERDs have demonstrated benefit in hormone receptor positive, HER2 negative metastatic breast cancer. SERDs appear to have few significant side effects. The currently approved SERD used in metastatic breast cancer is an injection. I-SPY 2 is testing a new oral SERD in early-stage patients. SERDs have been shown to be even more effective than aromatase inhibitors or tamoxifen for ER positive breast cancer.
Patients participating in the Endocrine Treatment Phase will be randomly assigned to receive either an investigational oral SERD (Amcenestrant) alone or in combination with another investigational targeted drug (Letrozole (AI) or Abemaciclib) for 6 months.
If you are a premenopausal or perimenopausal woman, you will also receive an oral ovarian suppression drug. This helps to prevent your body’s natural hormones from interfering with the study treatment.
Once you finish the treatment, you will have surgery to remove any tumor that may be left in your breast.
Information About Drugs Offered in the I-SPY 2 Trial
You can find more details about the different classes of investigational drugs used in the I-SPY 2 trial here.
Different classes of investigational drugs work in the body in different ways.
One class of investigational drugs that we have tested is PARP inhibitors. PARP inhibitors prevent tumor cells from repairing damaged DNA. Cancer cells that cannot repair DNA damage are more likely to die. PARP inhibitors are effective when used with another chemotherapy drug, such as carboplatin, that causes DNA damage. It is most effective in cells that already have a defect in the DNA repair mechanism such as tumor cells from patients with BRCA mutations.
Another class of investigational drugs being tested is called kinase inhibitors. Drugs belonging to this class interfere with the signaling mechanism that tells tumor cells to divide or die.
The I-SPY 2 trial is also testing immune-oncology investigational drugs such as PD-1/PD-L1 inhibitors. This class of investigational drug is showing promise in fighting many types of cancer. These block the cancer cell’s ability to protect itself, so the body’s immune system can destroy the cancer cells.
What should I do next?
As you are thinking about taking part in the I-SPY 2 Trial, you may find this trusted downloadable National Cancer Institute (NCI) booklet helpful, “Taking Part in Cancer Treatment Research Studies”.

You can print or email this I-SPY 2 Trial Patient Fact Sheet to family and friends so you can discuss your course of treatment and treatment decisions with them.
Now that you know more about what to expect from the I-SPY 2 Trial, meet the I-SPY 2 physicians and researchers and see them discuss the essential elements of the trial.
You can also hear from some I-SPY 2 trial participants as they discuss how they decided to participate and how they feel about their experiences on the trial.
![]() The I-SPY 2 Trial is sponsored by Quantum Leap Healthcare Collaborative, a 501(c)(3) charitable organization integrating high-impact clinical research with patient care.
The I-SPY 2 Trial is sponsored by Quantum Leap Healthcare Collaborative, a 501(c)(3) charitable organization integrating high-impact clinical research with patient care.
© 2023 Quantum Leap Healthcare Collaborative | Version 1.2 | All Rights Reserved.
![]()
The I-SPY 2 Trial is sponsored by Quantum Leap Healthcare Collaborative, a 501(c)(3) charitable organization integrating high-impact clinical research with patient care.
© 2023 Quantum Leap Healthcare Collaborative | Version 1.2 | All Rights Reserved.
